CO2 concentration and pH control in the cell culture laboratory
Most cell culture laboratories will have a humidified Carbon Dioxide (CO2) incubator set to 5% CO2, however it is not always obvious why our cultures need this amount of CO2 nor what the acceptable tolerances are for the ranges in CO2 concentration. You may also have heard or read that Dulbecco’s Modified Eagle’s Medium (DMEM) requires a higher (10%) concentration of CO2, yet most of us use 5% CO2 with this medium and there is scant information available to advise the impact of using lower CO2 concentrations with DMEM.
CO2 is not a metabolic requirement for cell cultures, its purpose is to dissolve into cell culture medium where a small proportion of it reacts with water to form carbonic acid which in turn interacts with its conjugate base (the dissolved bicarbonate ions in the medium) to control a stable physiological pH through the bicarbonate buffering system. The amount of sodium bicarbonate (NaHCO3) in the medium dictates the amount of CO2 that should be used to maintain the pH. Physiological pH is generally considered to be in the range of 7.2 to 7.4 for normal tissues, but some disease states (such as cancer) may require culture at a lower pH. Eagle’s Minimal Essential Medium (EMEM) supplemented with Earle’s Balanced Salt Solution (EBSS) and most other cell culture media are formulated with 26mM sodium bicarbonate, DMEM on the other hand has a far higher concentration of 44mM sodium bicarbonate.
The bicarbonate buffering system is not the most efficient chemical system for controlling pH. There are other synthetic buffers such as HEPES which work over a wider range of laboratory conditions. The bicarbonate system, however, occurs naturally in the human body and is therefore used in in vitro cell culture to mimic human and mammalian physiology, minimising toxic side effects.
Generally, cell culture media are also supplemented with the indicator dye Phenol Red. A yellow hue to the medium indicates the pH is too low (acidic), a purple hue shows the pH is too high (alkaline) whereas an orangey-red hue indicates the medium is of the correct physiological pH (Figure 1).

Figure 1. Cell culture media are generally supplemented with Phenol Red to act as a pH indicator. The colour of the medium helps advise if cultures are at physiological pH and can assist with troubleshooting.
Bicarbonate buffering works through Le Chatelier’s principle. Increased acidity in the medium is manifested by an increase in Hydrogen (H+) ions; free bicarbonate ions then react with the extra H+ ions to form carbonic acid “shifting the reaction to the left”, stabilising pH. (See Figure 2). Similarly, a decrease in H+ ions will result in a “shift to the right”.
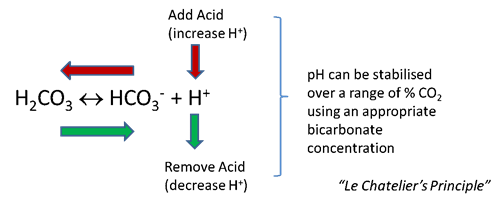
Figure 2. The principle of bicarbonate buffering
Using Dalton’s law of partial pressures to determine the partial pressure of CO2 in the incubator atmosphere, Henry’s law together with the Henry’s constant of the medium to determine the amount of CO2 that dissolves into the medium at 37°C and the Acid Equilibrium Constant of the bicarbonate buffering system it is possible to estimate the theoretical pH values that should be observed in different medium formulations at different percentages of CO2 and therefore evaluate the ranges of CO2 concentration that can be used for the media to maintain an optimal physiological pH range.
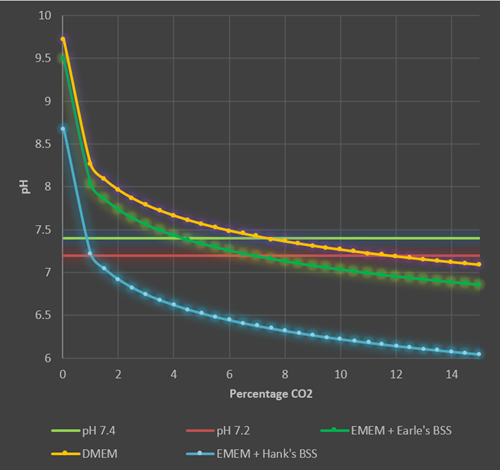
These calculations (see Appendix) can then be used to estimate the pH range seen in different culture media with different percentages of CO2 in the incubator (Figure 3).
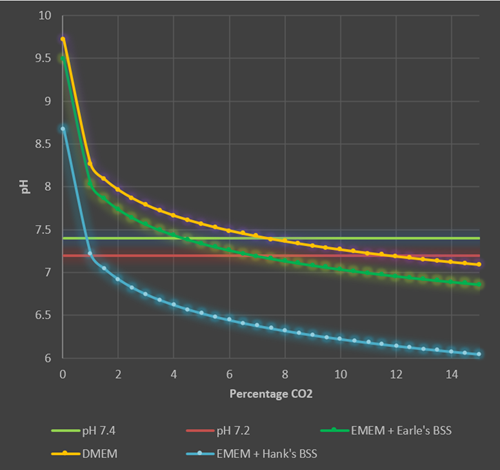
Figure 3. Theoretical pH ranges for different culture media in increasing CO2 levels in a 37°C humidified incubator. The lower and upper physiological range of pH is denoted by the red and green horizontal lines (pH 7.2-7.4). DMEM (44mM NaHCO3, orange line) is held at physiological pH at CO2 concentrations between 7.5% and 11%. Using DMEM in a 5% CO2 environment will result in a pH of 7.5 which although acceptable for most cell cultures is slightly outside the desired range of physiological pH. EMEM + Earle’s Balanced Salt Solution (BSS) (26mM NaHCO3, dark green line) is held at physiological pH with CO2 levels between 4.5% and 6.5%, whereas a much lower percentage of CO2 (near atmospheric levels) is required for EMEM + Hank’s BSS. (4mM NaHCO3, blue line).
These theoretical data show that for most cell culture media with 26mM NaHCO3, at 37°C the percentage of CO2 in the incubator should be maintained at a nominal 5% (between 4.5% and 6.5%). In theory DMEM (44mM NaHCO3) a higher percentage of CO2 should be used (between 7.5% and 11.5%, at a nominal level of 10%). However, it has become convention to use 5% CO2with DMEM and most cell line suppliers will advise this. Cell culturists should be aware that using 5% CO2 with DMEM will result in a pH of 7.5-7.6 which is slightly above the physiological range, however, in most cases the lactic acid and CO2 evolved by healthy, growing cultures will offset and correct this high pH. If you are working with very low density cultures in DMEM or have particularly slow growing cultures, then increasing the CO2 level of the incubator may be a worthwhile exercise. Bear in mind, however, that higher percentage of CO2 will displace oxygen in the incubator, reducing the available oxygen to the cells, so percentages of CO2 above 10% should not be used.
Finally, it is vitally important that CO2 incubators are properly serviced and calibrated to ensure that both temperature and CO2 displays are accurate. At ECACC we use an independent CO2monitor calibrated to United Kingdom Accredited Service (UKAS) Standards to regularly check and re-calibrate the internal incubator CO2 probe and display (Figure 4).

Figure 4. The Geotech G100 CO2 monitor. An example of a calibrated CO2 monitor that can be used to check and verify CO2 incubator internal probes and displays.
References
- Freshney, R. I. (2010) Culture of Animal Cells John Wiley & Sons, Inc.
- Johnson, Leonard R., ed. (2003). Essential Medical Physiology: Elsevier Academic Press.
Appendix: Calculations and Formulas Used Note:
- These calculations are based on Henry’s Law at 37⁰C for blood and assumes the Henry’s constant of complete culture medium to be similar.
- Only a very small proportion of the dissolved CO2 reacts to form carbonic acid, so Henry’s Law “holds true”.
- The system must run at atmospheric pressure.
- The calculations are based on cell culture medium alone. The effect of CO2 and lactic acid produced by cell cultures is not cot considered.
-
To determine the amount of dissolved CO2; use Henry’s Law
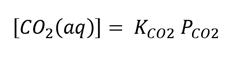
Where KCO2 is the Henry’s Constant of CO2 (3.0x 10-2 mole/L x Atm at 37⁰C) (blood/culture medium)and PCO2 is the partial pressure of CO2 in the incubator. Calculate the partial pressure of CO2 in Atmospheres (Atm) units:
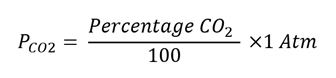
Then calculate the concentration of aqueous CO2 ([CO2 (aq)]) using Henry’s Law, and convert the concentration to Moles/L.
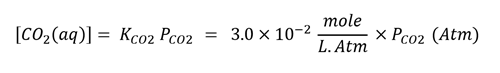
2. Now we have the [CO2 (aq)], using the acid equilibrium constant of the Carbonic Acid / Bicarbonate reaction we can estimate the hydrogen ion (H+) concentration.
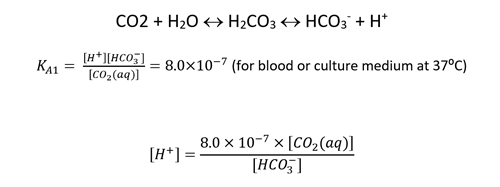
3. The pH can then be calculated:
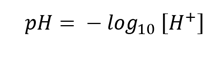
Jim Cooper
