How culture collections can assist responses to emerging disease
Diagnosis of viral infections is often achieved by testing clinical samples using molecular methods such as PCR and sequencing, or serology to detect circulating antibodies. Although these methods are relatively fast, inexpensive, and straightforward, they are targeted to specific pathogens, and can miss unexpected causes. You can only find what you look for. In contrast, although viral isolation is time-consuming and can require high biocontainment, it remains an essential aspect of a public health response. Biological material such as live virus is needed to develop and validate molecular and serological tests that are then used to detect and diagnose on a wider scale. Live virus is also needed to develop therapeutics, as challenge material in pre-clinical vaccine trials, and to conduct basic research into pathogenesis, transmission and vector competence. Therefore virus isolation is essential to increase preparedness for emerging diseases.
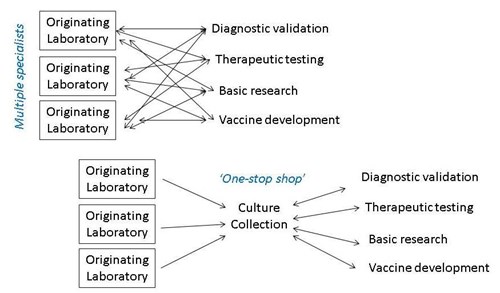
All these research and development activities require collaboration and sharing of virus strains between many participants, not just within the isolating laboratory. In addition, sharing of biological material is often required by publishers and funders. Unfortunately, transferring infectious material between scientific institutions has a high administrative burden. For example import and export licences and material transfer agreements may be required for each transfer. Dual-use, environmental, animal and plant pathogen regulations and intellectual property rights may also need to be navigated. Investigators may also be concerned about potential loss of characteristics or cross-contamination followed by onward transfer to third parties, which could lead to potential reputational damage for the originating institution. Finally, actual shipment of infectious cultures requires specialist knowledge of dangerous goods packing and shipping regulations. Delegating sharing to NCPV, by depositing your strain with us, addresses all of these concerns.
In Culture Collections of PHE, we have expertise in logistics and dispatch of dangerous goods. Our standard Terms & Conditions replace most material transfer agreement (MTA) requirements, and when a contract is still required we have a dedicated Business Development team. Intellectual property rights are retained by the depositor, and we have an accredited quality management system in place to authenticate every batch before it is released for supply.
Depositing with NCPV helps recipient researchers obtain material rapidly too. In cases of unusual, poorly studied or neglected pathogens, it can be difficult for end-users intending to validate a multiplex assay or perform basic research, to track down multiple specialist labs who may hold the strain of interest via their publication histories. Therefore it is more expedient to source all their material from a single well-known supplier. They can also be assured that the strains are independently authenticated, rather than risk using potentially misidentified strains.
Culture Collections: A one stop shop
Although deposits can be made at any time, ideally they would be made before an outbreak occurs. This allows time for many of the required permits to be obtained, and physical stock of virus batches are prepared and quality controlled before high-throughput dispatch is required. As an example, Zika virus strain MP1751 was held at Porton Down since 1962. In 2013 a freezer was being cleared of unwanted material, but a vial of the then-obscure Zika virus was wisely deposited with NCPV. In 2015 patients started testing positive for Zika virus in Brazil, and a ProMed alert prompted NCPV to prepare a batch of virus and establish authentication protocols. In November our local diagnostic laboratory asked us for a sample of Zika virus as they were required to start testing travellers returning to the UK from South America. Shortly afterwards a batch was available for supply, and NCPV was the only source in the world of authenticated Zika virus. When the World Health Organisation declared the Zika virus outbreak a Public Health Emergency of International Concern in February 2016, laboratories all over the world required live Zika virus in order to develop and validate surveillance and screening tests. As growth protocols had already been established the previous year, NCPV stocks were easily replenished with no real interruption to supply.
Zika virus deposit timeline
A study recently published by the Johns Hopkins Centre for Health Security identified that respiratory-transmissible RNA viruses were most likely to cause a global pandemic, but other possibilities should not be ignored. The World Health Organisation blueprint for disease priorities includes Disease X, the acknowledgment that a pandemic may be caused by a pathogen currently completely unknown.
In summary,
- Live pathogens are needed for preparedness and response to emerging diseases
- Sharing of biological material is necessary, but requires a lot of administration
- Depositing strains with NCPV allows faster, more efficient sharing
- Deposits can be made before, during or after an outbreak
- Pandemics cannot be accurately predicted, so a broad range of pathogens should be continuously deposited, as part of an effective ongoing surveillance program
To learn about the process of depositing, visit How to deposit strains with NCPV.
Reference
“The Characteristics of Pandemic Pathogens” by Johns Hopkins Center for Health Security www.centerforhealthsecurity.org/our-work/pubs_archive/pubs-pdfs/2018/180510-pandemic-pathogens-report.pdf
Written by Karen Buttigeig, NCPV Lead
