Optimisation of the cryo-preservation of chick embryo fibroblast cells (CEF): viability alone does not indicate the recoverability potential of cryo-preserved CEF cells
Summary
Chick embryo fibroblasts (CEF) are a useful cellular reagent in virology as they support the growth of many human and animal pathogenic viruses. This study has helped ECACC establish reproducible cell banks of CEF that can be used directly for virus propagation and titration without the need for reverting to primary tissue preparation. It also illustrates the fact that cell viability, although a useful measure of cryopreserved cell quality, is not a guarantee of efficient recovery of cell cultures from frozen.
Introduction and Methods
Chick embryo fibroblasts (CEF) are useful cellular reagents in virology as they support the growth of many human and animal pathogenic viruses, such as influenza, enabling diagnosis, in vitro viral amplification, and titration of viral stocks1. Many scientists prefer the convenience of working with frozen reproducible stocks of cells (cell banks) rather than having to generate cultures from primary tissue whenever cells are required2. However, historically, we have found cryopreservation of CEF problematic.
In an attempt to overcome this problem we theorised that the concentration and nature of the cryo-protectant was critical in the cryo-preservation process for CEF cells. To test this theory we prepared freeze mixtures containing four Dimethylsulphoxide (DMSO) concentrations (1,2, 5, and 10% v/v) in foetal bovine serum (FBS). We cryopreserved the cells obtained from primary isolations in these media using a rate controlled freezer (Planer) programmed to produce an optimised cooling profile from 4⁰C to liquid nitrogen temperature. Following cryo-preservation the vials were stored in a Dewar for several days at liquid nitrogen temperature before resuscitation and plating into tissue culture flasks. Cell counts and viabilities were determined on triplicate samples from each DMSO concentration using the Nucleocounter NC 200® viability assay (Chemometec). The attachment and growth of the cells was assessed automated cell imaging (Incucyte Zoom®, Essen Bioscience) using the increase in percentage confluency over time as a measure of recovery.
Results
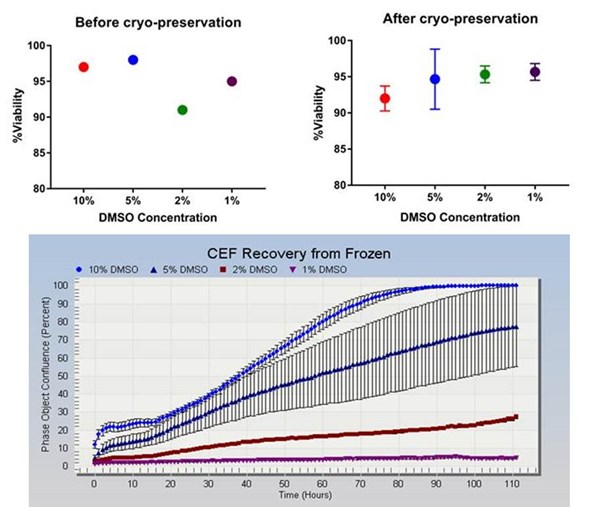
The figures show that cell viabilities pre and post cryo-preservation were over 90% for all conditions (top left and top right) and equivalent in all conditions. The recovery and growth of the cells is shown in the lower graph with the best results seen in cells cryopreserved in 10% DMSO. Error bars represent standard deviation. The wider spread of error bars for the 5% DMSO treatment was attributed to a high degree of variability in confluence in these cultures.
Discussion and Conclusions
In order for any cell line to be a useful reagent in in vitro studies it is imperative that it can be efficiently and reproducibly cryo-preserved and recovered from frozen. It is important that cells have high viabilities at the point of freezing and this high viability is maintained throughout the cryo-preservation, storage and recovery process but it is also essential that cryopreserved cells establish proliferative cultures in an suitable time frame after thawing as demonstrated by rapid attachment, spreading and increasing confluence . Here we have shown that high viabilities can be maintained in cryopreserved CEF using a range of DMSO concentrations however with these particular CEF cells, 10% DMSO proved to be the optimum concentration of cryo-protectant to achieve efficient cell recovery. Lower concentrations of DMSO, although maintaining high viabilities did not allow the full recovery of cultures.
