Application notes
 You can access our application note by clicking on the link below:
You can access our application note by clicking on the link below:
Written by: Jim Cooper and Dr Simone Lilley, Culture Collections, the UK Health Security Agency (UKHSA)
Introduction
ECACC cDNA is supplied in a convenient easy to use format providing a “snapshot” of cellular expression from log phase cultures for use in comparative Quantitative Real Time PCR (QRT-PCR) gene expression experiments.
|
ECACC Number |
Cell line name |
Description |
|---|---|---|
|
VCaP |
Human prostate cancer metastasis. Cosmic sample COSS2580131 Prostate (Carcinoma; Adenocarcinoma) |
|
|
PC-3 |
Human prostate adenocarcinoma. COSS1998469 Prostate (Carcinoma; Adenocarcinoma) |
|
|
PNT1A |
Normal human post pubertal prostate immortalised with SV40 |
|
|
PNT2 |
Normal human prostate immortalised with SV40 |
|
|
SerBob |
Spontaneously immortalized human prostate cancer (requires foetal bovine serum (FBS)) |
|
|
Shmac 1 |
Prostate cancer |
|
|
Shmac 4 |
Prostate cancer moderately well differentiated, early stage |
|
|
Shmac 5 |
Prostate cancer moderately well differentiated, early stage |
|
|
P4E6 |
Prostate cancer well differentiated, early stage |
|
|
LNCap clone FGC |
Human Caucasian prostate carcinoma. Cosmic sample COSS2580128 Prostate (Carcinoma; Adenocarcinoma) (LNCap) |
|
|
Bob |
Spontaneously immortalized human prostate cancer (serum-free) |
|
|
22Rv1 |
Human prostate xenograft Cosmic sample COSS1689707 Prostate (Carcinoma; Adenocarcinoma) |
|
|
PNT1A (SERUM FREE) |
Normal human prostate immortalised with SV40 (serum free) |
|
|
PNT2 (SERUM FREE) |
Human prostate normal, serum-free |
Table 1. The ECACC Prostate Cell Line Panel
Androgen Receptor and Prostate Cancer
There are around 11,500 prostate cancer deaths in the UK every year. It is the 2nd most common cancer in UK males, accounting for 13% of all cancer deaths in men. Since 2005 prostate cancer mortality rates have decreased by 12%1. In most cases, prostate cancer cells metastasize and spread to bones and this is the main cause of death. The mechanisms that underlie this process are not fully understood2.
The standard treatment for metastatic prostate cancer is to reduce circulating male specific androgen hormones by surgical or chemical castration. However, in most, if not all patients, a non-treatable aggressive castration resistant prostate cancer (CRPC) will eventually emerge causing relapse.
The androgen receptor (AR) is highly expressed in cells of patients in CRPC3 and it seems that the receptor plays a role in disease progression4. Prostate cancer cell lines with or without AR expression are useful tools for the generation of in vitro and animal models. Historic reports and anecdote imply that the cell line PC-3 is negative for the expression of AR but it has recently been reported that this cell line may express AR at low levels5.
ECACC has a panel of 14 different prostate cell lines (see Table 1). The aim of this work was to use cDNA from the panel to compare the relative expression of AR expression by QRT PCR and to then confirm AR protein expression using immunofluorescence.
For prostate cancer cell lines included in the Sanger Catalogue of Somatic Mutations in Cancer (COSMIC) database6 where detailed genomic analysis data are available, the COSMIC sample ID and description is also included in Table 1.
QRT PCR
cDNA samples from proliferating (log phase cultures) were provided by ECACC together with reverse transcriptase-free controls which were confirmed absent of genomic DNA carry-over by QRT-PCR in accordance with MIQE guidelines7.
cDNA samples were stored at -20°C until required at which time they were thawed on wet ice and diluted 1:10 in nuclease-free water to provide working material for the QRT-PCR assays. QRT-PCR for AR was set up twice, in duplicate by two different scientists on separate occasions using TaqMan probe based PCR using curated primers for AR (Thermo Fisher). AR Ct values were obtained using the QuantStudio 7 (Applied Biosystems) with standard TaqMan cycling conditions and normalised to the geometric means of the CTs from four stably expressed reference genes (ACTB, GAPDH, B-2M and TOP1). Relative gene expression was calculated using the Delta-Delta Ct method8. We used cDNA from the human hepatocellular carcinoma cell line Huh-7D12 (ECACC 01042712) as a negative control and the expression level of AR in LNCap Clone FGC as positive control and comparative baseline.
Results
|
Cell line name |
Positive or Negative for AR Expression by QRT PCR |
Level of expression |
|---|---|---|
|
VCAP |
Positive |
Highest |
|
LNCAP Clone FGC |
Positive |
High |
|
22RV1 |
Positive |
Medium |
|
Bob |
Positive |
Low |
|
PNT1A Serum Free |
Positive |
Low |
|
SHMAC5 |
Positive |
Low |
|
PC-3 |
Positive |
Very Low |
|
SHMAC1 |
Positive (high Ct values) |
Very Low |
|
PNT1A |
Negative |
- |
|
PNT2 |
Negative |
- |
|
SERBob |
Negative |
- |
|
PE46 |
Negative |
- |
|
PNT2 Serum Free |
Negative |
- |
Table 2. The hierarchy of AR gene expression in the ECACC prostate cell line panel as determined by QRT PCR.
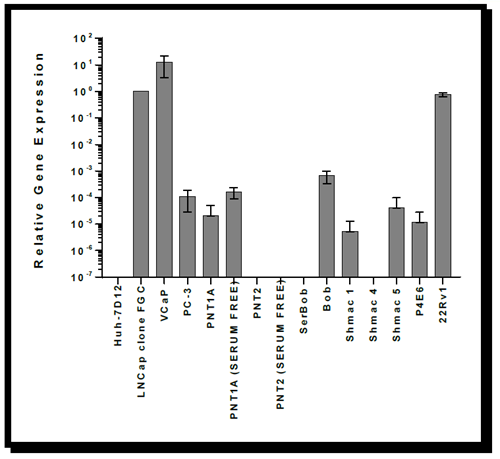
Figure 1. Graph showing the relative gene expression of Androgen Receptor mRNA in ECACC prostate cell lines as normalised to the geometric means of four stably expressed reference genes (ACTB, GAPDH, B-2M and TOP1). NB: Y axis has been log transformed for visual purposes. Gene expression is relative to that observed in LNCap clone FGC.
Confirmation of AR expression by Immunofluorescence
Our QRT-PCR data obtained from the cDNA panel showed that PC-3 cells (ECACC 00112714) may express AR at low levels and confirmed that the cell line LNCaP Clone FGC (ECACC 8911021) expressed high levels of AR9. The liver cell line Huh-7D12 was used as a negative control and as expected show no evidence of AR expression. These three cell lines were cultured using the prescribed media and conditions as advised by ECACC on coverslips and stained for AR expression using the AR411 mouse monoclonal antibody (Thermo Fisher MA5-13426) and a goat anti-mouse Alexafluor 488 labelled secondary antibody (Thermo Fisher R10477). The actin cytoskeletons of the cells were counterstained with Alexafluor 647 phalloidin (Molecular Probes).
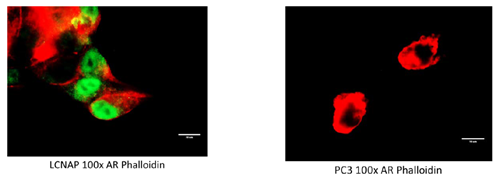
Figure 2. LNCaP Clone FGC cells expressed nuclear androgen receptor (AR) (green staining, left) whereas PC-3 cells were negative for AR expression (right). Red staining: actin cell cytoskeleton (phalloidin).
Conclusions
-
QRT PCR of the cDNA transcripts of a panel of 14 ECACC prostate cancer cell lines showed a hierarchy of gene expression for AR as shown in Table 2.
-
VCaP (ECACC 06020201) and LNCAP Clone FGC cells displayed the highest levels of AR expression of the panel, however VCaP has a long population doubling time and may not be suitable for some cell culture experiments10.
-
These results concur with the literature in that the PC-3 was positive for AR expression by QRT-PCR analysis but at a very low level. AR expression in these cells was not demonstrated by immunofluorescence (IF). This was likely to be due to the level of expression being below the limit of detection of the IF technique.
-
The cell lines Bob and SerBob were derived from the same patient11. Bob was cultured in complex serum-free medium (Keratinocyte-SFM (Invitrogen, catalogue no 37010-022, with prequalified human recombinant epidermal growth factor 1-53 (EGF 1-53), bovine pituitary extract (BPE) and glutamine) + 2ng/ml leukaemia inhibitory factor + 2ng/ml stem cell factor + 100ng/ml cholera toxin + 1ng/ml granulocyte macrophage colony stimulating factor) whereas Serbob was cultured in serum containing medium and is reported to be more invasive than its serum free counterpart. In this experiment, Bob (serum-free) was shown to be AR positive, whereas Serbob (serum-cultured) was negative for AR expression.
-
Results also indicated the tentative possibility that the cell line PNT1A (Serum free) cultured in a similar serum free media to Bob may express more AR than its serum-cultured counterpart.
-
cDNA from the panel of ECACC prostate cell lines proved to be useful resource for the characterisation of AR expression and demonstrates that the cDNA product is beneficial in the screening of cell lines for expression of genes of interest
References
1. Prostate cancer statistics. Cancer Research UK (2015). Available at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer. (Accessed: 5th March 2019)
2. Jin, J.-K., Dayyani, F. & Gallick, G. E. Steps in Prostate Cancer Progression that lead to Bone Metastasis. Int. J. Cancer J. Int. Cancer 128, 2545–2561 (2011).
3. Ruizeveld de Winter, J. A. et al. Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am. J. Pathol. 144, 735–746 (1994).
4. Donovan Michael J. et al. Androgen receptor expression is associated with prostate cancer‐specific survival in castrate patients with metastatic disease. BJU Int. 105, 462–467 (2009).
5. Alimirah, F., Chen, J., Basrawala, Z., Xin, H. & Choubey, D. DU-145 and PC-3 human prostate cancer cell lines express androgen receptor: implications for the androgen receptor functions and regulation. FEBS Lett. 580, 2294–2300 (2006).
6. Cosmic. COSMIC - Catalogue of Somatic Mutations in Cancer. Available at: https://cancer.sanger.ac.uk/cosmic. (Accessed: 27th March 2019)
7. Bustin, S. A. et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 55, 611–622 (2009).
8. Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, research0034.1-research0034.11 (2002).
9. Horoszewicz, J. S. et al. LNCaP Model of Human Prostatic Carcinoma. Cancer Res. 43, 1809–1818 (1983).
10. Korenchuk, S. et al. VCaP, a cell-based model system of human prostate cancer. Vivo Athens Greece 15, 163–168 (2001).
11. Attard, G. et al. A novel, spontaneously immortalized, human prostate cancer cell line, Bob, offers a unique model for pre-clinical prostate cancer studies. The Prostate 69, 1507–1520 (2009).
