A guide to using UKHSA LENTICULE® disc Norovirus GI/GII and Hepatitis A virus Reference Materials (RMs)
 Refer to the Material Safety Data Sheet and the Certificate of Analysis for more specific details about the individual UK Health Security Agency (UKHSA)
Refer to the Material Safety Data Sheet and the Certificate of Analysis for more specific details about the individual UK Health Security Agency (UKHSA)
LENTICULE® disc product, available online here.
-
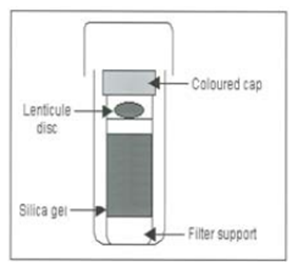
On receipt store the LENTICULE® discs in the small plastic vials at –20°C ± 5°C
-
LENTICULE® discs are reconstituted by a process of re-hydration and dispersion. Remove the vial(s) to be used from freezer storage at -20°C ± 5°C and allow them to reach room temperature for approximately 5 to 10 minutes before use
-
Tap the cap of the vial to ensure the LENTICULE® disc is located on top of the silica gel desiccant. Open the vial and transfer the LENTICULE® disc either by using fine forceps or by inverting the vial into a tube containing 1 ml ± 0.1 ml of sterile phosphate buffered saline (PBS)
-
Leave for at least 10 minutes at room temperature to re-hydrate. Ensure that the disc is completely dissolved before proceeding
-
Follow your routine laboratory testing procedure for RNA extraction and determination
-
If your methods enable determination of genome copies per LENTICULE® disc compare your results with the Certificate of Analysis. If your laboratory methods differ from those used to
generate the reference values you are advised to carry out calibration using your routine method
-
The recommended storage temperature for LENTICULE® discs is -20ºC ±5ºC. Storage temperatures below -30ºC may alter the balance of this matrix which can affect the subsequent recovery of organisms from the discs
-
Transport of LENTICULE® discs from the provider does not require temperature controlled conditions; viability is not normally affected by ambient temperatures encountered during transit
Problem solving advice for virus LENTICULE discs
Reconstitution of the LENTICULES®
a. Ensure that the LENTICULE® has dissolved fully in the phosphate buffered saline (PBS) diluent. The disc should be allowed to dissolve completely before proceeding. Shaking the tube will speed up the dissolving process but will result in some frothing. If frothing occurs allow the bottle to stand until this disappears
b. Ensure that the PBS is at a concentration of NaCl 8.0g, KCl 0.2g, Na2HPO4 1.15g KH2PO4 0.2g per litre, pH 7.3 and if in tablet form has been made up in molecular grade water
c. Ensure that the PBS is within its expiry date.
Extraction of viral RNA
a. Viral RNA should be extracted from the same volume of reconstituted LENTICULE® as would be extracted using your routine method.
b. Ensure that the extraction buffers are formulated correctly and within the assigned expiry date.
c. Extracted RNA should be stored ≤-15°C before downstream processing.
Real time PCR
a. Ensure that the primer and probe sequences are correct, and that they have been reconstituted correctly
b. Ensure that the PCR buffer recipe is correct and that the buffer has been made up accurately
c. Ensure that sample RNA and PCR buffer have been added to the correct wells
d. The equipment should be operating within specification, and the temperature, time and labelling parameters should be appropriate for the target assay.
Results
a. Results should be checked for failures in reagents or equipment leading to inefficient RT-PCR
b. Ensure that there is no cross contamination or laboratory introduced contamination of reagents
c. Adjust the threshold value to an appropriate level based on the strength of the signal and the background curves
d. Check that the curves are sigmoidal.
